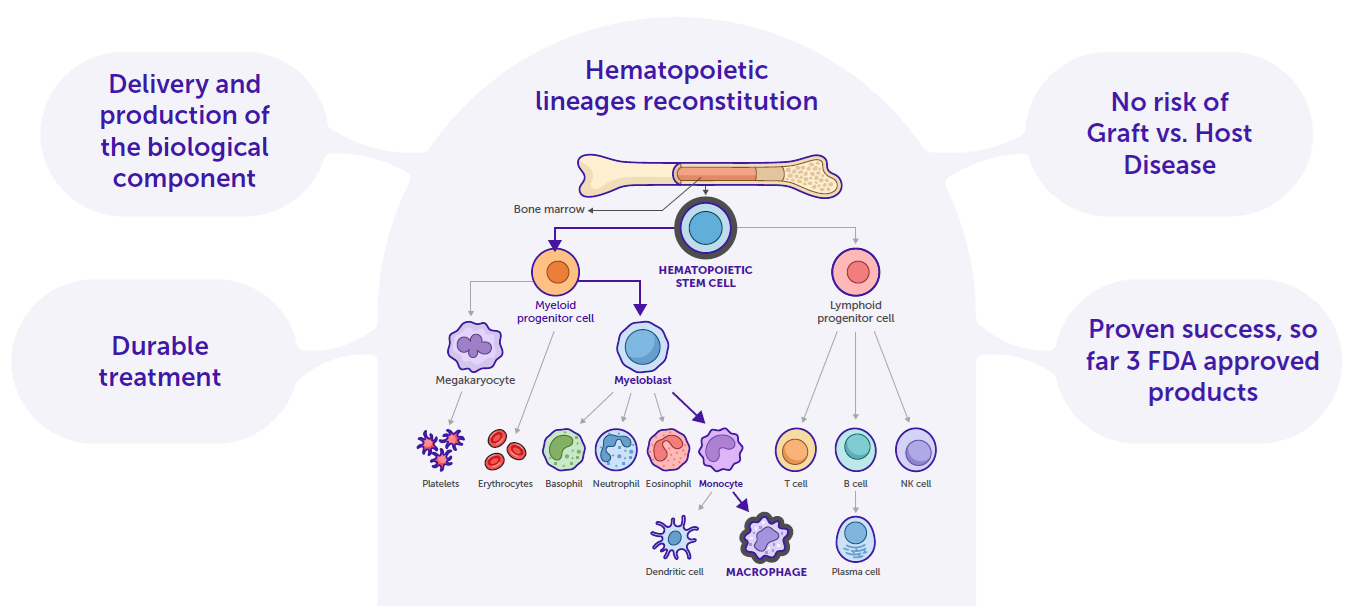
Our use of hematopoietic stem cells (HSCs) builds on one of the safest and most effective forms of gene therapy, leveraging their role as the foundation of the entire hematopoietic system. HSCs naturally differentiate into multiple cell lineages, each playing vital roles in the body. By harnessing the immune system’s inherent ability to connect and regulate the body, we create therapies that are precise, durable, and transformative for patients with rare and severe conditions.
This approach is grounded in the validated technology of genetic reprogramming of blood stem cells, which has already been used to successfully cure several genetic disorders. Years of research and development in lentiviral vector engineering have made this possible. By replacing essential lentiviral genes with human genes of interest, these engineered viral vectors can transduce HSCs, integrating the desired genes directly into the cell’s chromosomes. This innovative method reprograms the genetic code, enabling the production of therapeutic proteins and transforming the way complex diseases are treated.
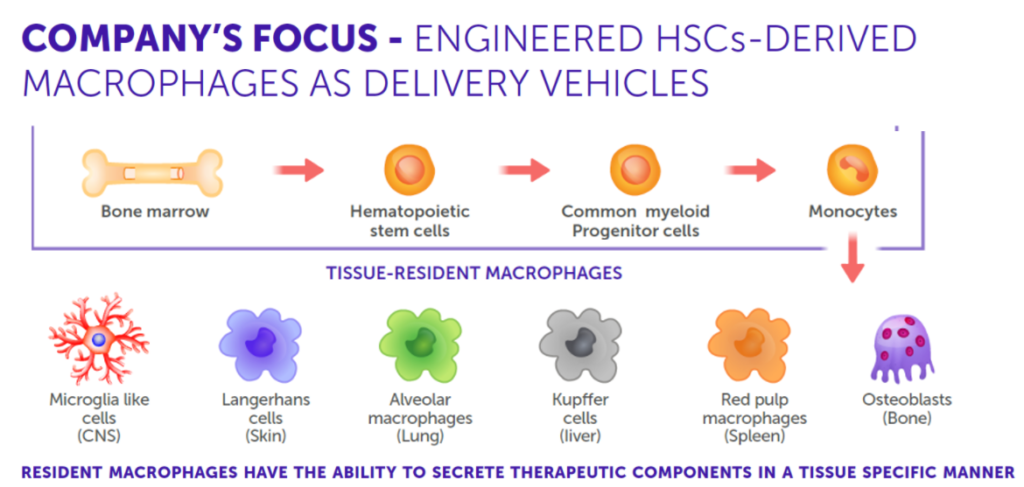
Tissue-resident macrophages, a specialized subset of immune cells, exhibit unique gene expression profiles that allow them to adapt to and thrive in their specific tissue environments. These properties make them exceptional candidates for delivering biological therapies. By engineering these macrophages with smart expression cassettes, we enable them to secrete therapeutic molecules in a sustained and localized manner, offering a transformative approach to treating a wide range of diseases

Noga’s strong partnership with LONZA, a global leader in cell and gene therapy manufacturing, allows us access to Cocoon™, a state-of-the-art cell and gene therapy manufacturing system.
Performing the process of genetic reprograming of stem cells with lentiviruses in Cocoon™ ensures the robustness, precision and reproducibility of manufacturing. This reduces the time and cost of the personalized production of the therapy for each patient.

A 22-year entrepreneur who founded a Nasdaq-listed company and has invested in
numerous businesses. Currently leads a family office. Holds a B.Sc. in Business Administration and Economics from Tel Aviv University.




Mr. Adam Brian heads Lonza Personalized Medicine, which focuses on developing and bringing to market tools that enable research and development, scale out, and commercialization of patient scale cell therapies, with an emphasis on decentralized and point of care manufacturing. Mr. Brian is a results-oriented leader with extensive experience in the life science and medical device industries. Proven track record of increasing revenue, improving operations, and building and leading high-performing teams.

Dr. Eytan Abraham heads Resilience cell, gene and nucleic acids Franchises. Dr. Abraham holds a Ph.D. in developmental and molecular biology from the University of Maryland Biotechnology Institute, and a post-doctorate in cell-therapy and tissue engineering from the Harvard-MIT Biomedical Engineering Center and Harvard Medical School. Dr. Abraham is an experienced scientist and business leader with expertise in basic and applied biological and cell therapy R&D as well as in management, technology, and commercialization.

An internationally recognized physician-entrepreneur, executive and investor, with over 20 years of clinical and industry healthcare experience. Founder at MedExplore Ventures and of a number of HealthTech/Biotech Companies. Previously, General Partner at a HealthTech PE fund and VP At Eli Lilly.




Dr. Yeal Weiss is currently CEO of Mahzi Therapeutics, a company focused on the development of therapies for ultra-rare genetic neurodevelopmental disorders. Dr. Weiss completed her MD at Hadassah Medical School at the Hebrew University in Jerusalem and her PhD at the Weizmann Institute of Science in Rehovot, Israel. She has over 20 years of industry experience in medical/clinical and business development roles at Genzyme, Merck and Ultragenyx. Dr. Weiss is a member of the NIH driven Bespoke Gene Therapy (BCTG) consortium, ASGCT translational committee, N=1 collaborative and is a 2022 Termeer Fellow. Board member/advisor to ADNP and FOXG1 foundations.

20 years of experience in complementary medicine, former head of the Chinese Medicine Unit at Sheba Hospital. Founder of the Association for Noga (Her Way), An advocate and director in the rare disease world. Holds a BSc. From Brighton University.


Extensive experience in cell and gene therapy. Led the recently approved hemophilia A program at Spark Therapeutics and the earlyphase oncology development at Enlivex Therapeutics. Holding a PhD from the Technion.




10 years of experience in leading R&D projects in the industry, with extensive understanding of gene & cell therapy, holding a PhD from the Weizmann institute.

